AVITI/Illumina (Short-Read) Sequencing Sample Requirements
The MSU Genomics Core accepts user-prepared libraries and prepares DNA-seq, RNA-seq, and amplicon libraries for short-read sequencing on the AVITI from Element Biosciences. Libraries that are compatible with Illumina sequencers are also compatible with the AVITI. The requirements for these services are listed in the tables below.
The quantities specified below apply to samples that have been quantified by a fluorometric method, such as Qubit. The Genomics Core has a Qubit 1.0 which is available for self-service quantification in our Common Use Equipment room. Please see the Common Use Equipment Room webpage to reserve a time to use the Qubit 1.0. The Genomics Core also offers a Qubit Flex Quantification service for 72 or more samples. Please see the Qubit Flex Quantification Sample Requirements webpage for more information on this service.
If your samples do not meet the sample submission requirements, please contact the Genomics Core (gtsf@msu.edu) to discuss your specific situation.
User Prepared Library Requirements
Amplicon/Metagenomic Requirements
User Prepared Library Requirements
Submit libraries in nuclease-free water, Qiagen EB buffer, or 10mM Tris, 0.1% Tween20. Submit libraries in 1.5 ml microcentrifuge tubes, tightly sealed and clearly labeled with the sample name on the top and side of tube. If samples do not meet the requirements below, please contact the Genomics Core (gtsf@msu.edu) to discuss your samples prior to submission.
Please contact us if you need assistance determining the nanomolar (nM) concentration of your libraries.
| Instrument | Concentration | Minimum Volume |
|
AVITI |
≥ 15 nM |
30 µl Additional volume may be |
DNA Requirements
Submit DNA in nuclease-free water, Qiagen EB buffer, or 10mM Tris pH 8.0. All DNA must be RNase-treated.
If samples do not meet the requirements listed, please contact the Genomics Core (gtsf@msu.edu) prior to sample submission.
Libraries are compatible with AVITI and Illumina sequencers.
| Library Name | Use | Concentration | Volume |
| Quantabio sparQ DNA Library |
Standard Shotgun DNA Library Preparation1 |
≥ 15 ng/µl |
≥ 20 µl |
| Low Input DNA Library Preparation1 |
≥ 1 ng/µl |
≥ 20 µl |
|
| ChIP-seq Library Preparation2 |
≥ 1 ng/µl |
≥ 20 µl |
|
|
IDT xGen Methylation-Sequencing DNA Library1 (formerly Swift BioSciences Accel-NGS Methyl-Seq DNA Library) |
Methyl-Seq DNA Library Preparation. Whole Genome Bisulfite Sequencing (WGBS) and Reduced Representation Bisulfite Sequencing (RRBS) are possible. Contact the Genomics Core to discuss RRBS. Bisulfite conversion performed using Zymo EZ DNA Methylation-Gold Kit. | ≥ 15 ng/µl | ≥ 20 µl |
1 Genomic DNA must be high molecular weight and pure. Genomic DNA should be run on a gel to confirm integrity. Please provide gel image at the time of submission.
2 Average length of DNA fragments must be ≤ 450 bp. Provide TapeStation trace (or equivalent) at the time of submission to confirm size.
RNA Requirements
Submit RNA in nuclease-free water, Qiagen EB Buffer, or 10mM Tris pH 8.0. All total RNA samples must be DNA-free. RNA extraction protocols using TRIzol are not recommended. If a TRIzol method is used, a spin/clean-up column should be used to ensure any residual TRIzol has been removed.
A TapeStation trace or equivalent (such as Bioanalyzer) must be submitted with samples. Bioanalyzer RIN or TapeStation RINe scores of 8.0 or greater are recommended for non-plant samples and for non-photosynthetic plant samples. For photosynthetic plant samples that are run on the Bioanalyzer, RIN scores of 7.0 or greater are recommended. However, for photosynthetic plant samples that are run on the TapeStation, plastid rRNAs confound the TapeStation algorithm that calculates the RINe score, and RINe scores of about 5.0 may still represent largely intact RNA. Questionable TapeStation results from photosynthetic plant samples should be sent to the Genomics Core (gtsf@msu.edu) for review. See this Agilent Application Note for a description of the complications from plastid rRNA.
If samples do not meet the sample requirements listed below, contact the Genomics Core (gtsf@msu.edu) prior to submission.
Libraries are compatible with AVITI and Illumina sequencers.
| Library Name | Use | Concentration | Volume |
|
Watchmaker mRNA Library |
Standard mRNA library preparation. Appropriate for high quality, eukaryotic |
≥ 20 ng/ul |
≥ 20 µl |
|
Watchmaker RNA Library with FastSelect rRNA depletion |
FastSelect rRNA depletion kits are species specific. Please contact the Genomics Core (gtsf@msu.edu) to discuss your project. |
≥ 25 ng/µl | ≥ 20 µl |
| Lexogen QuantSeq 3' mRNA-Seq Library FWD | Quant-Seq generates highly strand-specific libraries of reads from the 3' end of each mRNA in your sample. Only one read is created from each mRNA, and many fewer reads are required for gene expression analysis. | ≥ 25 ng/µl | ≥ 15 µl |
| Lexogen Small RNA Library |
Library preparation of small RNAs (<200 nt), including microRNA (miRNA). Input is total RNA or enriched small RNA. |
≥ 25 ng/µl |
≥ 15 µl |
Amplicon/Metagenomics Requirements
The RTSF Genomics Core will include a negative, no template control (NTC) reaction on each plate of samples processed for amplicon sequencing. Researchers will have the option to request that a positive control be included with 16S-V4 projects. For more details, please see the Amplicon/Metagenomics guide.
The table below provides an overview of the submission requirements for 16S-V4 and all other amplicons (for barcoding). Additional requirements, PCR recipes, and cycling conditions are provided below the table.
16S-V4 Amplicons
Amplicon Barcoding
References
| Target | Input | Concentration | Volume | Multiplex | Notes |
| 16S-V4 | Metagenomic DNA | ≥ 1 ng/µl | ≥ 10 µl | 24 to 380 samples (unique dual-indexes) | Test amplification and gel image required, see below for more details. |
| Amplicon Barcoding | Primary PCR product | ≥ 1 ng/µl | ≥ 10 µl | 24 to 380 samples (unique dual-indexes) | Gel image required, see below for more details. |
16S-V4 Amplicons
The library preparation for the 16S-V4 region employs a one-step PCR method using the primer pair 515f/806r:
16S V4 forward (515f): GTGCCAGCMGCCGCGGTAA
16S V4 reverse (806r): GGACTACHVGGGTWTCTAAT
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and environmental microbiology 79, 5112–5120 (2013) (https://github.com/SchlossLab/MiSeq_WetLab_SOP/blob/master/MiSeq_WetLab_SOP.md)
The submitter is required to:
- Provide a minimum of 24 samples per project.
- Quantify samples via fluorometric method (such as Qubit or PicoGreen) and normalize all samples to approximately the same concentration.
- Perform a test amplification using the 16S-V4 primers (above) to confirm the sample can be amplified and that the product is the expected size. The PCR products should be run on a 2% agarose gel. A 100 bp ladder must be run alongside the samples so that the PCR products can be sized. The gel must be run long enough to have good separation of the ladder. The gel image(s) must be provided at the time of submission. The name and part number of the ladder must also be provided.
- Submit samples in a 96-well PCR-type plate, filled by column, starting with A:1. Well H:12 must be left empty for the RTSF no template negative control.
The Genomics Core will use 1 µl of genomic DNA for the amplicon library preparation. Therefore, it is recommended that test amplifications be performed using a protocol similar to the Genomics Core's amplicon library preparation protocol:
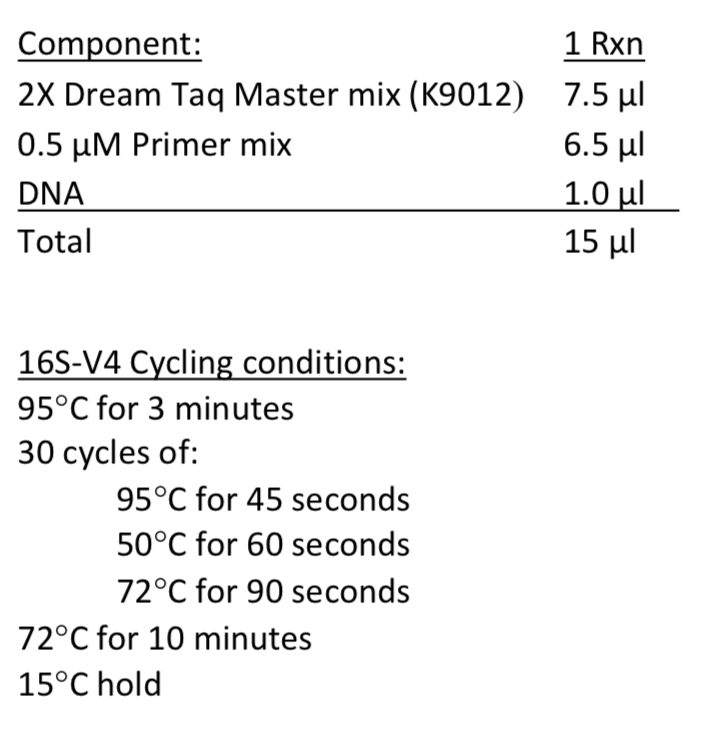
Amplicon Barcoding (index adapters added by MSU Genomics Core)
For all other amplicon sequencing, a two-step PCR approach is used. The first PCR uses target-specific primers with tags on the 5’ ends that allows the Genomics Core to do a second PCR for barcoding. The submitter is required to perform the primary PCR reaction. To do this, the submitter will need to order primers with the following tags on the 5’ ends, with the target-specific forward and reverse sequences inserted in [TS-For] and [TS-Rev], respectively:
CS1-TS-F: 5’ – ACACTGACGACATGGTTCTACA – [TS-For] – 3’
CS2-TS-R: 5’ – TACGGTAGCAGAGACTTGGTCT – [TS-Rev] – 3’
When choosing primers for a targeted amplicon it is recommended that the product amplified is less than 400bp (not including the 44bp added by the CS1/CS2 overhangs). This size will permit adequate overlap of the forward and reverse read 3' ends when sequencing with a 2x250bp format.
The submitter is required to:
- Provide a minimum of 24 samples per project.
- Quantify samples via fluorometric method (such as Qubit or PicoGreen) and normalize all samples to approximately the same concentration.
- Run products on a gel to confirm the amplification was successful, the products are the expected size, and that off-target products (including primer-dimer) are not present. The PCR products should be run on a 2% agarose gel. A 100 bp ladder must be run alongside the samples so that the PCR products can be sized. The gel must be run long enough to have good separation of the ladder. The gel image must be provided at the time of submission. The name and part number of the ladder must also be provided.
- Products do not need to be cleaned up prior to submission. However, if primer dimers or off-target products are generated, then products should be cleaned up to remove these products and/or the primary PCR reaction should be optimized.
- Submit samples in a 96-well PCR-type plate, filled by column, starting with A:1. Well H:12 must be left empty for the RTSF no template negative control.
The Genomics Core will use 1 µl of primary PCR product for the amplicon indexing
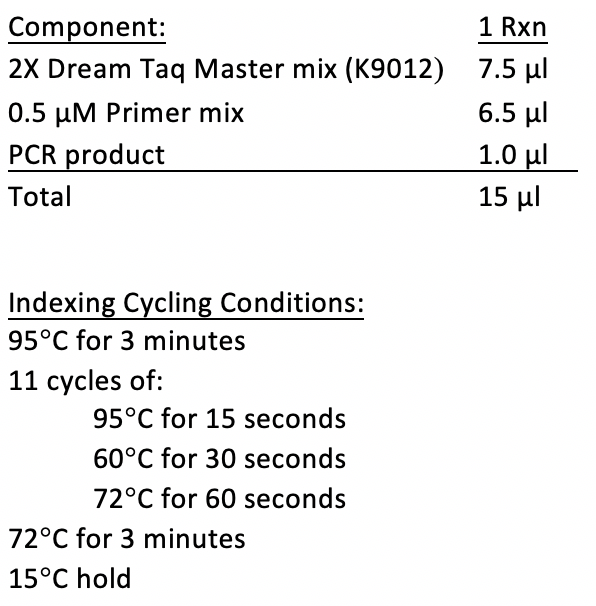
References
Kozich, J. J. et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and environmental microbiology 79, 5112–5120 (2013).
Klindworth, A. et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41, e1–e1 (2013).